• Analysis Spotlight
Fragile X syndrome is a genetic dysfunction attributable to the gene FMR1. It’s the most typical type of inherited mental incapacity and sometimes co-occurs with different situations like autism and epilepsy.
New analysis has unveiled main insights into genetic mechanisms underlying FXS and associated problems. The examine discovered that the problems contain in depth silencing of many genes that play key roles in constructing tissues and making the mind work correctly.
The analysis was funded by the Nationwide Institute of Psychological Well being, the Nationwide Institute of Neurological Issues and Stroke, the Eunice Kennedy Shriver Nationwide Institute of Youngster Well being and Human Improvement, and the NIH Frequent Fund’s 4D Nucleome Program.
How do modifications to the FMR1 gene result in FXS?
The prevailing principle is that FXS entails modifications to FMR1, a gene situated on the X chromosome (one in every of two intercourse chromosomes in people). The primary a part of the FMR1 gene is made up of repeats of a selected DNA sequence referred to as a CGG. The CGG sequence can increase uncontrollably, resulting in an extra variety of repeats. A standard-length FMR1 sequence has lower than 40 CGG repeats. In distinction, a mutation-length FMR1 sequence has over 200 CGG repeats.
When the FMR1 CGG reaches this mutation size, it causes bodily modifications to the gene that silence its expression. Because of this, FMR1 produces solely a little bit or none of its protein, which is required for wholesome mind operate. That is when FXS emerges. Folks with FXS missing the FMR1 protein can expertise vital impacts on their growth , together with mental and studying disabilities; speech and language difficulties; and social and behavioral issues, corresponding to hyperactivity and nervousness.
Nonetheless, modifications in FMR1 alone don’t account for the complete vary of FXS signs, as evidenced by mouse fashions during which the Fmr1 gene is “knocked out,” or genetically turned off. Earlier analysis has additionally implicated a broader set of genetic mechanisms and gene places in FMR1 silencing.
What did researchers do within the present examine?
Jennifer Phillips-Cremins, Ph.D. , and the examine’s first authors, Thomas Malachowski, M.S., Keerthivasan Chandradoss, Ph.D., Ravi Boya, Ph.D., and Linda Zhou, M.D., Ph.D., on the College of Pennsylvania led researchers in trying past the usual mannequin of FXS. They examined whether or not human FXS tissues present modifications within the genome (the whole set of genetic materials [DNA]) or the epigenome (chemical modifications that affect gene expression with out altering the DNA sequence) and whether or not these modifications are particular to FMR1 or have an effect on different genes as properly.
The researchers mixed a number of high-tech analytic strategies, together with molecular mapping, DNA imaging, epigenetic sequencing, and genetic engineering. Extra computational approaches allowed them to combine and discover patterns within the massive datasets they used.
The researchers examined genetic, epigenetic, and imaging information in a number of human cell traces from individuals with FXS and other people with out the dysfunction. They appeared genome-wide for modifications occurring on each the X chromosome and non-sex chromosomes (often called autosomes ). As well as, they labored with the NIH NeuroBrainBank to gather postmortem tissue from a mind space linked to FXS—the caudate nucleus—from individuals with and with out the dysfunction.
These superior strategies enabled the researchers to take a look at the form of the DNA in the whole genome and the way it folds into complicated 3D constructions contained in the cell nucleus (often called the 3D genome). The folding of the genome in 3D area displays the packaging and interactions of segments of chromatin (the mixture of DNA and protein that makes up the genome). Exact folding patterns within the 3D genome are important for correct gene regulation and mobile operate, and deviations from this construction are related to genetic problems like FXS in addition to many cancers.
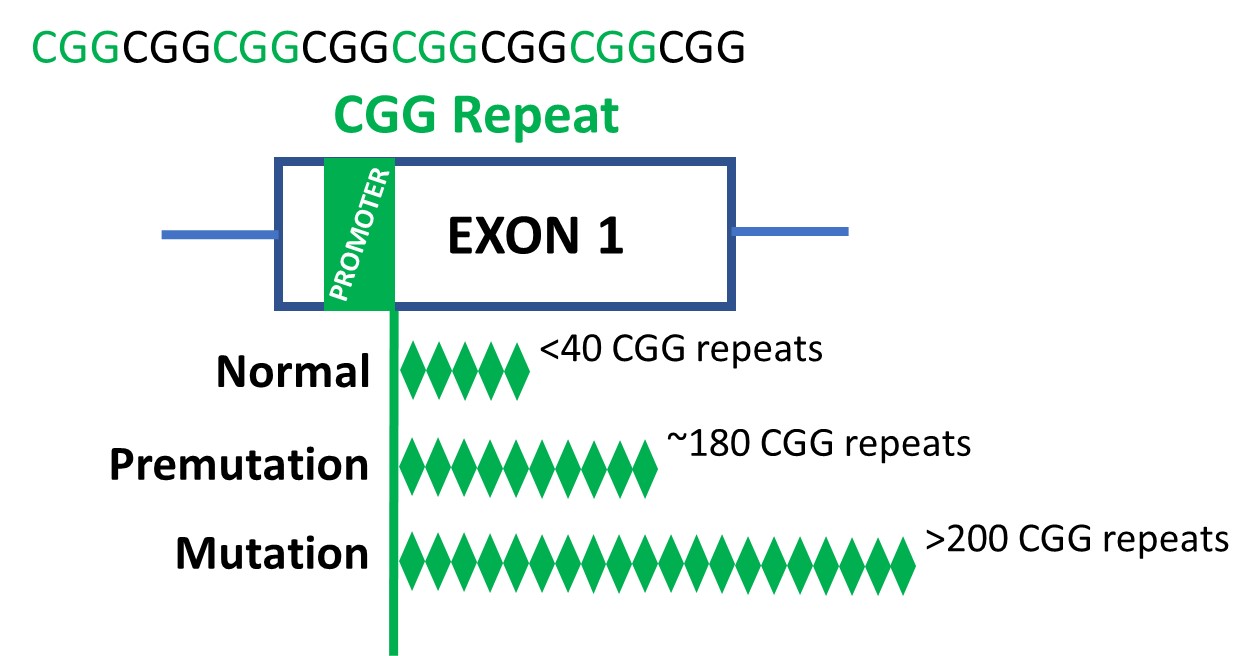
In all cell traces and tissues, the researchers in contrast the three variations of theFMR1 CGG repeat that may happen:
- Regular size (5–45 CGG repeats)
- Premutation size (61–199 CGG repeats)
- Mutation size (>200 CGG repeats; FXS)
By analyzing the 3D genome and chromatin modifications decided by the size of the CGG repeat, the researchers might discover epigenetic modifications and hyperlink them to gene expression silencing and genome instability that may end up in genetic problems.
What did the outcomes present?
The outcomes confirmed, in cells and tissue from individuals with FXS, beforehand identified modifications related to the mutation-length CGG growth, together with epigenetic modifications to the FMR1 gene and silencing of FMR1 gene expression. The researchers additionally unexpectedly found extra widespread modifications to the DNA.
They discovered deposits of enormous pockets of a silencing type of chromatin, often called heterochromatin, that’s folded too tightly and makes the gene much less accessible. The heterochromatin pockets prolonged far past FMR1. On the X chromosome, the heterochromatin radiated outward to silence genes upstream of FMR1 which can be important for neural cell capabilities corresponding to circuit connectivity and synaptic plasticity, which might trigger studying difficulties generally skilled by individuals with FXS. On autosomal chromosomes, the heterochromatin silenced a number of genes associated to pores and skin, tendon, and ligament integrity, that are clinically affected tissues in individuals with FXS.
The researchers additionally discovered extreme misfolding of DNA and attainable websites of breakage alongside the DNA sequence or slight expansions of different repetitive sequences inside the heterochromatin. Most of these increased order genome folding patterns inside cells are important for correct gene operate, so the recognized modifications in genome construction are probably related to FXS past the native silencing of FMR1.
The Cremins Laboratory coined the time period beacons of repeat growth anchored by contacting heterochromatin, or BREACHes, to discuss with the community of silenced gene areas they found, marked by extreme chromatin misfolding and genome instability. The researchers suggest that the organic significance of BREACHes is silencing genes concerned in processes important for mind operate, together with ones controlling synaptic plasticity that allows the mind to alter in response to studying and different dynamic conditions. Silencing of those genes might assist clarify most of the developmental variations seen in individuals with FXS.
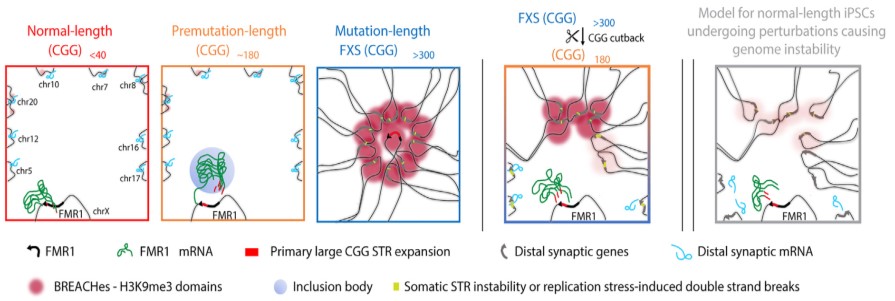
Having established broad structural and useful modifications attributable to the mutation-length FMR1 CGG, the researchers checked out what occurred after they in the reduction of the repeat to premutation size. They discovered that shortening the CGG size led to the removing of heterochromatin on most of the BREACHes. This discovering signifies that the size of the CGG repeat is a vital contributor to a minimum of a number of the extreme and widespread 3D genome misfolding and gene silencing seen in FXS and associated problems. It additionally means that reverse-engineering the mutation-length CGG repeat might probably forestall the emergence of genome-wide defects seen in widespread genetic problems in people.
What do the outcomes imply?
Collectively, the outcomes reveal vital modifications within the genome construction associated to the mutation-length FMR1 CGG sequence. These modifications, related to issues in how the DNA folds and instability within the genome, induced genes with essential roles in mind operate to change into silent or inactive, serving to clarify most of the signs seen in individuals with FXS.
The silenced areas spanned a community of widespread gene places that the researchers referred to as BREACHes. BREACHes have been noticed in a number of cell sorts and postmortem mind tissue from individuals with FXS and on each the X chromosome and a number of autosomal chromosomes. BREACHes additionally included a number of genes not beforehand linked to FXS, providing new targets for future investigation.
In response to the researchers, the invention of BREACHes fills a serious lacking piece in understanding FXS. The novel discovering helps clarify the medical presentation and frequent signs of individuals with FXS, which couldn’t beforehand be defined by lack of the FMR1 protein alone. As a result of genome misfolding occurred not solely in FXS cell traces but additionally in cells with instability generally, BREACHes could be significant to the rising listing of problems marked by unstable repeat expansions.
This examine enhances understanding of the genetic and epigenetic mechanisms contributing to FXS illness pathology. The outcomes might, in time, have broad relevance for understanding, diagnosing, and even treating the various problems marked by unstable repeat expansions or genome instability, together with FXS and most cancers.
Reference
Malachowski, T., Chandradoss, Okay. R., Boya, R., Zhou, L., Cook dinner, A. L., Su, C., Pham, Okay., Haws, S. A., Kim, J. H., Ryu, H.-S., Ge, C., Luppino, J. M., Nguyen, S. C., Titus, Okay. R., Gong, W., Wallace, O., Joyce, E. F., Wu, H., Rojas, L. A., & Phillips-Cremins, J. E. (2023). Spatially coordinated heterochromatinization of lengthy synaptic genes in fragile X syndrome. Cell, 186(26), 5840–5858. https://doi.org/10.1016/j.cell.2023.11.019
Grants
MH120269 , MH129957 , DK127405 , DA052715 , HD098015 , NS129317